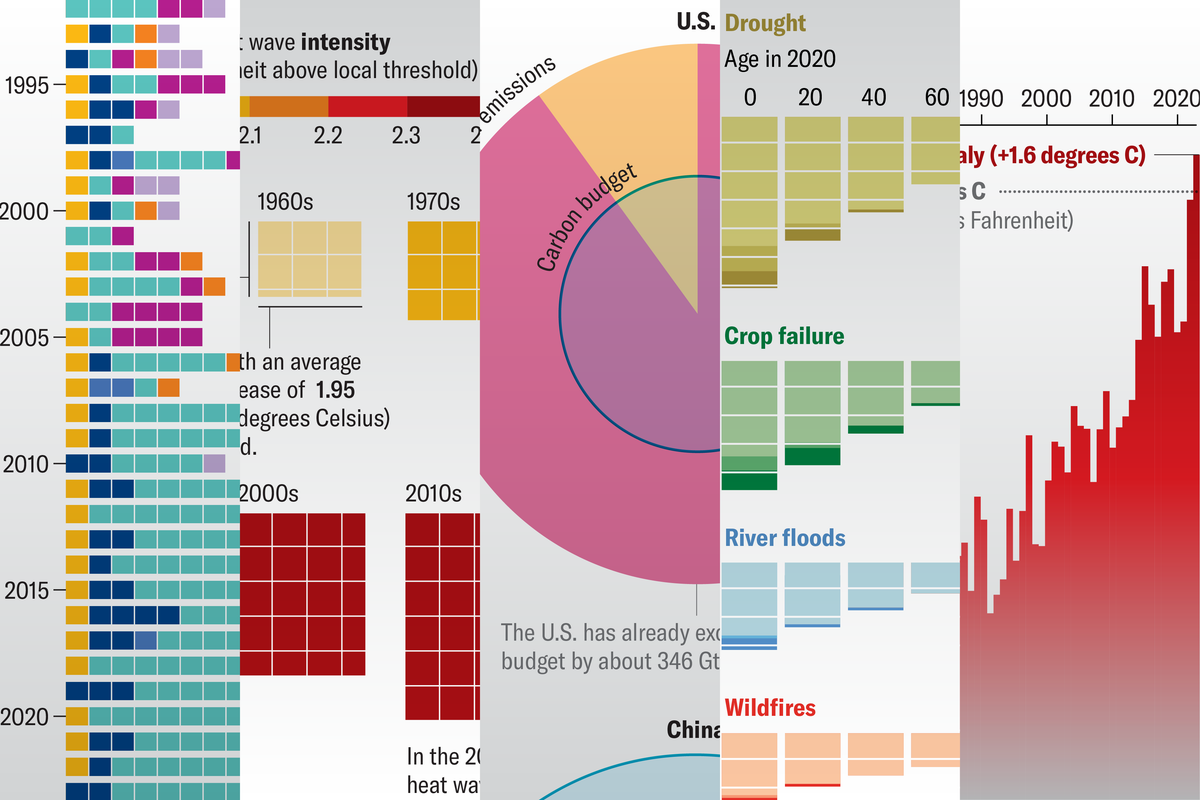
Minks in Spain, seals in Scotland, sea lions and dolphins in South America: a number of mammal species have recently been found to be infected with H5N1, a highly pathogenic strain of avian influenza. Avian flu is not new; epidemiologists have been studying it for decades. But the detection of the virus in mammals has many concerned about the potential that it could spill over to humans and cause a larger outbreak.
As the world enters the fourth year of a global pandemic caused by a virus that likely came from an animal, concern over another virus potentially uprooting our lives is valid. H5N1 has infected humans before, although human-to-human transmission has rarely been observed. And while the World Health Organization (WHO) reports that the mortality rate of avian flu in humans is around 56 percent, many experts believe it’s likely to be much lower if the virus becomes more transmissible. One reason avian flu is so lethal is that it infects the lower respiratory tract, which can lead to respiratory failure. If it were to mutate to infect the upper respiratory tract and spread more easily, it would probably cause milder illness. Yet even a virus that causes mild or moderate illness in many people can have a serious toll, as we’ve seen with the COVID pandemic. So efforts are underway to develop vaccines to protect against such a form of bird flu.
Bird flu has spilled over to humans before—in fact, just last week a girl in Cambodia died from H5N1 (although not the same strain as the one that is sickening birds worldwide).
“Each time we see this happen, we get these spurts of cases, [and] people say, ‘Here it comes; it’s going to happen,’” says Michael Osterholm, director of the University of Minnesota’s Center for Infectious Disease Research and Policy. But he stressed that H5N1 is likely not an imminent threat to humans.
Others agree. “I’m keeping a close eye on it as an expert, but as a member of the community, as a parent and someone who [has been] recently experiencing the COVID-19 pandemic, I’m not worried about this right now,” says Caitlin Rivers, an epidemiologist at the Johns Hopkins Center for Health Security. “This is an animal health issue right now that has a theoretical risk to become a human health issue.”
Avian influenza viruses infect birds by attaching to a receptor in their respiratory tract. In order for the virus to become a human virus and start circulating from person to person in the population, it would have to be able to attach itself to the human version of this receptor—something it hasn’t yet evolved to do.
“It’s a really dangerous time to be a bird,” Andrew Pavia, chief of the division of pediatric infectious diseases at the University of Utah, adds. “But as of today, the risk to humans remains very low. Our concern is what’s going to happen as it circulates more and more.”
It’s unfortunately nearly impossible to predict when this jump could happen. “None of us know when the next influenza pandemic will emerge. It could be tomorrow [or] it could be years from now, and we don’t know which of the viruses will become the next pandemic virus,” Osterholm says. “At the outset, you have to say there is uncertainty, with one exception: there will be a pandemic.”
The U.S. is somewhat equipped to handle avian flu: there is a stockpile of egg-based flu vaccines for the H5N1 strain. Eggs are one of the most common ways to make a flu vaccine. To create it, manufacturers inject an inactivated or weakened virus into a fertilized chicken egg, incubate the egg for a few days while the virus multiplies and then harvest the virus to use for the vaccine. The country has a secret chicken stockpile in undisclosed locations across the U.S. just in case we need to make egg-based vaccines quickly—such as during a flu pandemic. It may be concerning that this vaccine strategy depends on an animal that is highly susceptible to the flu in question. But several experts told Scientific American that there are high levels of biosecurity at the chicken facilities in order to avoid bird flu contamination.
Alternatives to egg-based vaccines exist. Since the early 2010s, the U.S. Department of Health and Human Services has been partnering with CSL Seqirus, one of the largest influenza vaccine manufacturers, to develop vaccines grown in cells in a laboratory. The pandemic preparedness vaccine AUDENZ, which targets the H5N1 subtype specifically, is approved by the Food and Drug Administration. And although it’s impossible to determine which virus will cause the next pandemic, CSL Seqirus has a library of viruses that have the potential to infect humans. And the company is constantly looking for candidate vaccine viruses that it can tailor to a specific pathogen.
“We use data [for] one of the avian strains to create a mock-up file,” says Marc Lacey, who runs CSL Seqirus’s pandemic preparedness and response team. The team members basically identify certain viruses, use them to create vaccines and conduct some of the early safety studies so that if an avian flu strain evolves into a true pandemic strain transmitting between humans, they will be ready to go with an adequate vaccine. Lacey says that his company would be able to supply the U.S. government with 150 million doses within the first six months of a pandemic being declared, but he thinks the scale-up potential could be higher, especially if multiple manufacturers could help produce it. Because there are eight billion people in the world, scale-up and widespread collaboration across countries would be necessary to make enough vaccine.
There are also efforts to apply messenger RNA (mRNA) technology—such as that used for some of the COVID vaccines—to flu vaccines. According to University of Washington microbiologist Deborah Fuller, these efforts range from developing a universal flu vaccine to creating a “multivalent” vaccine that targets only a few subtypes—or versions of the virus (like a typical seasonal flu vaccine does). One advantage of mRNA technology is its speed of production. And because of the COVID pandemic, there is now more infrastructure to mass-produce doses. “RNA vaccines can be designed extremely quickly—you only need the genetic sequence of what the new variant is that’s emerging, and within weeks, [you] can have a vaccine already tested in animal models,” Fuller says.
Scott Hensley, a professor of microbiology at the University of Pennsylvania, is also investigating mRNA flu vaccines. He’s part of a research team developing a 20-subtype mRNA flu vaccine that includes an H5N1 strain (although not the one currently circulating in birds). The team recently published its findings in Science. Hensley’s lab is now developing a single-strain vaccine tailored to the current bird flu strain—and is already testing it in laboratory animals. He emphasizes that, as with the COVID vaccines, his vaccines are meant to prevent serious illness and death, not infection.
But even the 20-subtype vaccine is expected to provide some protection against the new strain. “When we developed [that] vaccine, the idea was to create one that could induce a certain level of immune memory against every subtype,” Hensley says. “Our goal wasn’t to predict which influenza subtype would cause the next pandemic or which strain would cause it. Rather the vaccine [would induce] some level of immune memory against every subtype so that it would limit disease and death caused by new pandemic strains.”
A vaccine is only part of pandemic preparedness—you also need effective treatments. As Pavia says, researchers aren’t working from scratch trying to create something like the COVID antiviral Paxlovid, which took nearly two years to get to market. FDA-approved antivirals for influenza, such as Tamiflu, already exist and will be important for reducing deaths during a flu pandemic. Pavia is also hopeful that the U.S. could quickly adapt diagnostic tests to match an outbreak strain of pandemic flu.
“We have better tools and better knowledge about influenza than we did about coronaviruses. In many ways, we would be a step ahead,” Pavia says. “But what I worry about is our political capacity to respond. We need to continue to [increase] awareness and continue preparation efforts. And if things start to accelerate, we need a concerted, nonpoliticized effort to move quickly and effectively.”