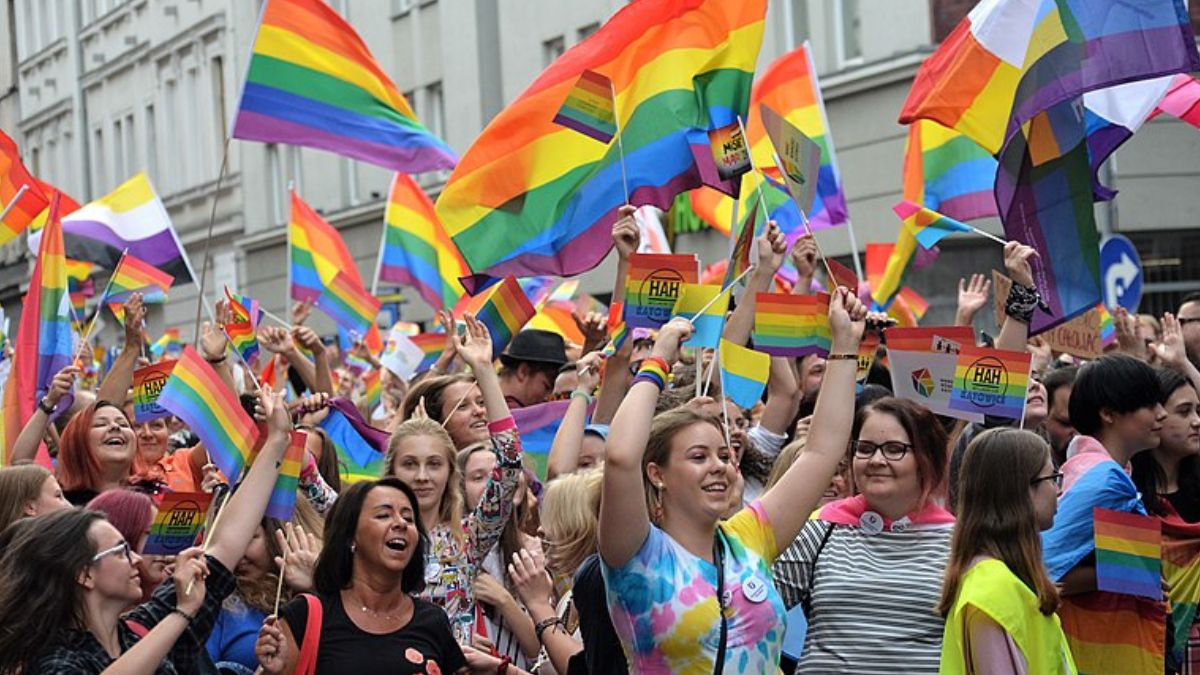Unlike existing treatments that focus on rehabilitation or reducing the risk of an additional stroke, the experimental drug targets the “stickiness” that can prevent injured brain tissue from functioning as it should
Health
28 July 2022
A 3D magnetic resonance imaging (MRI) scan of the brain of a 65-year-old after a stroke. The orange colour represents dead tissue SOVEREIGN, ISM/SCIENCE PHOTO LIBRARY
A drug that improves motor skills and memory in mice after the most common form of stroke may one day fill an unmet need in treatment for this debilitating condition.
After an ischaemic stroke, when the oxygen supply to the brain is temporarily blocked by a blood clot, treatments focus on rehabilitation or drugs that reduce the risk of future strokes. No approved drugs work to repair brain tissue that is left damaged. These injuries can cause widespread complications, including poor memory, movement issues and a reduced ability to solve problems.
In 2009, researchers at Case Western Reserve University in Ohio identified a protein receptor that binds to so-called chondroitin sulphate proteoglycans (CSPGs), which are abundant in scar tissue. CSPGs can create a “stickiness” that prevents repair of damaged brain tissue.
Now, the researchers have developed a drug that targets this CSPG receptor. “Our drug prevents this stickiness so the tissue can be repaired,” says Jerry Silver at Case Western Reserve University.
To test the drug, which is a peptide, for stroke damage, Silver and his colleagues injected 40 mice daily with the peptide or saline solution for three weeks, starting one week after the rodents had a surgery-induced ischaemic stroke.
Next, the team measured the time it took for the mice to escape a maze that they learned to navigate pre-stroke. The mice that received the drug solved the maze more than twice as quickly, on average, compared with those given saline injections, suggesting the treatment improved their memory.
In a second part of the experiment, the researchers stuck a piece of tape onto one paw of each mouse. Those treated with the drug removed the tape with another paw and their mouth more than twice as fast as the control mice, suggesting an improvement in fine motor skills.
“After the treatment, they’re cognitively improved, their motor skills are improved and this is when we treat them from not just one day, but a full week after injury – that’s pretty cool,” says Silver.
When the team imaged the brains of the mice, they found that those given the peptide had an increased number of connections between their neurons. Brain stem cells had also migrated into the injury site, forming new neurons.
“The results are surprising, given the delivery [an injection under the skin in the lower back] and the delay of the [first] drug administration [one week post-stroke],” says Stanley Thomas Carmichael at the University of California, Los Angeles.
Although not tested, the treatment may also work in other forms of stroke.
“The system that is influenced by this peptide is also induced in other forms of stroke and in brain haemorrhage, so one might imagine that it will have activity in these other stroke types,” says Carmichael.
The team hopes to gauge drug dosing by testing it in healthy volunteers soon. The peptide was found to be safe in early Phase I clinical trials for spinal cord injuries.
“The [body-wide] delivery and the fact that these molecules, chondroitin sulphate proteoglycans, are also induced in human stroke is promising for translation to humans,” says Carmichael.
Journal reference: Cell Reports, DOI: doi.org/10.1016/j.celrep.2022.111137
More on these topics: