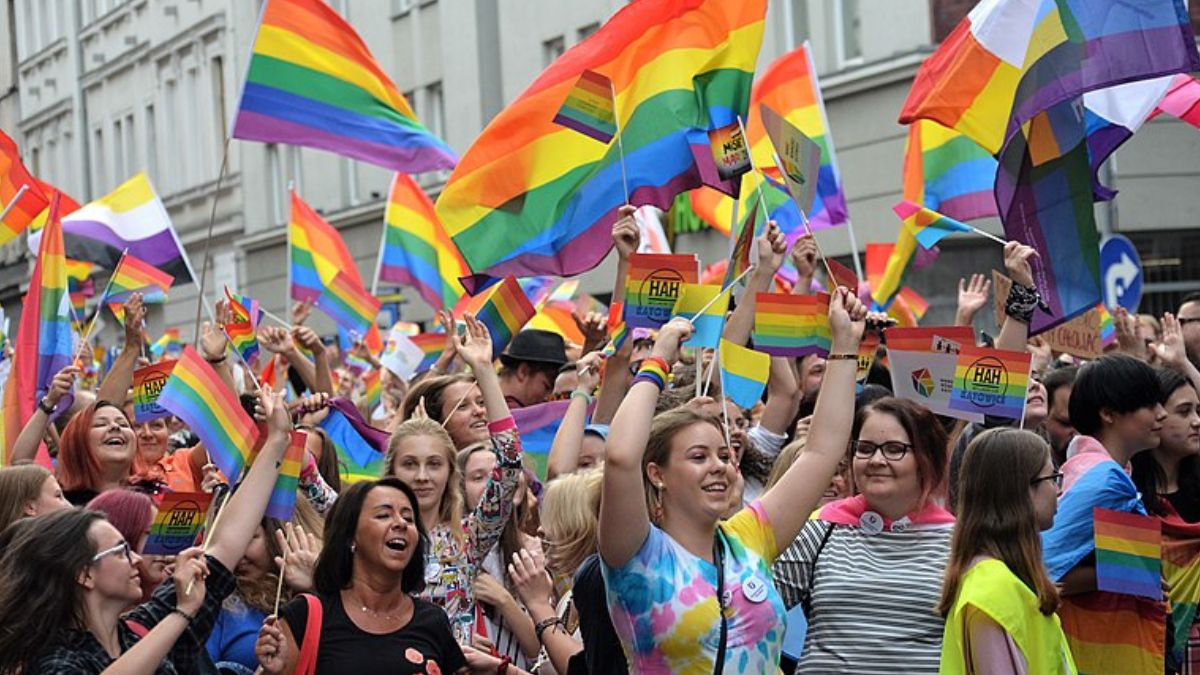Exactly how clouds produce rain is a mystery
Jack Dykinga/naturepl.com/Alamy
Moments after supercooled water freezes, a strange kind of spring-like ice is born. The process involved, which researchers have only now seen for the first time, could help explain how clouds, which contain millions of supercooled water droplets, make rain and affect Earth’s climate.
Clouds are made up of many tiny drops of water at temperatures below freezing. These can exist as liquid until they are penetrated by an ice particle, which kick-starts a complex and poorly understood succession of freezing states. The length and frequency of these different states are crucial for models that simulate how certain clouds make rain and reflect light in the atmosphere, but they happen so fast that they are difficult to study.
Now, Claudiu Stan at Rutgers University–Newark in New Jersey and his colleagues have discovered a kind of ice that forms inside supercooled water droplets which is both compressed and stretched at different points, like a spring in motion, microseconds after it first freezes. “It’s something that was definitely unexpected for us,” says Stan. “It took us a while to understand.”
To capture this ice and the overall freezing process, Stan and his team dropped a stream of water droplets through a vacuum which cooled them to around -39°C (-38°F). They then used both a microscope and X-rays to image tens of thousands of these droplets. Although they only had one image for each droplet at a particular stage of freezing, they could map out the entire process by looking at many, a bit like watching a flipbook animation.
The researchers found that each droplet turns into a slush ball, with a network of ice permeating the liquid water, before freezing fully from the outside inward. This ratchets up the internal pressure, until the droplet either shatters or squirts out water, both of which result in ice particles that can freeze other droplets. This, combined with the kind of ice formed, might better explain how and when these droplets form ice in clouds that turns to rain, although the lab environment is too different for the results to be directly applied, says Stan.
Finding this strained ice doesn’t fit with our current molecular-level understanding, says Stephen Cox at the University of Cambridge. “Trying to understand the molecular mechanisms of ice formation is important across many fields, from climate science to food technologies. This study demonstrates that we still have a long way to go, and I expect it to stimulate lots of new research in this area.”
Topics: