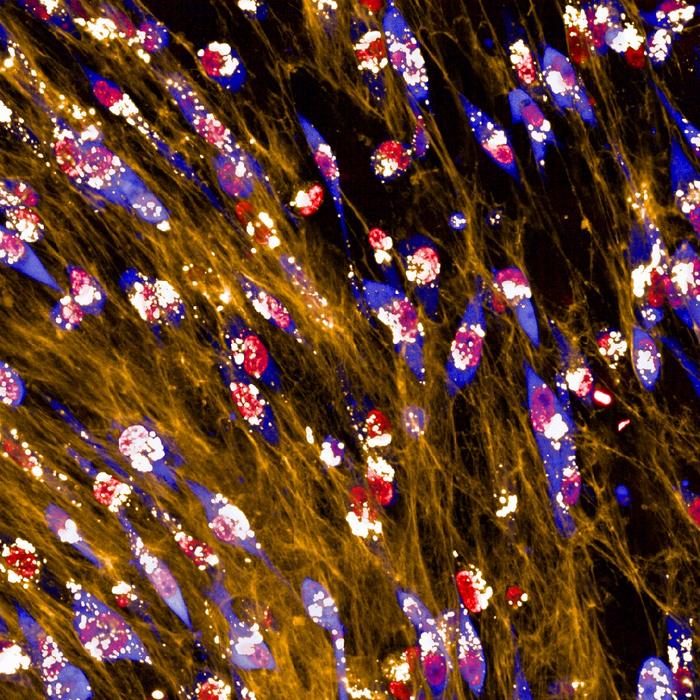
Breast cancer cells ingest and consume the matrix surrounding them to overcome starvation, according to a new study publishing January 16th in the open access journal PLOS Biology, by Elena Rainero of the University of Sheffield, UK, and colleagues. The finding elucidates a previously unknown mechanism of cancer cell survival, and may offer a new target for therapy development.
Cells in the breast, including tumor cells, are embedded in a meshwork called the extracellular matrix (ECM). Nutrients are scarce in the ECM, due to limited blood flow, and become even scarcer as tumor cells grow. And yet they continue to grow, leading the authors to investigate how tumor cells supply themselves with the raw materials to support that growth.
To do so, they seeded breast adenocarcinoma cells into either collagen (a major component of the ECM) or a commercial matrix preparation, or onto plastic, with or without certain critical amino acids. Without those amino acids, cells on plastic fared poorly compared to those in one or the other matrix. Similar results were seen with other matrix models—the tumor cells were able to overcome the reduction of amino acids when surrounded by matrix.
Next, by fluorescently labeling the collagen and watching its journey through the cell, the authors showed that the cells took up ECM and broke it down in digestive compartments called lysosomes; when the ECM was chemically treated to cross-link its components, the cells were unable to ingest it. Further investigation indicated that uptake was through an ingestion process called macropinocytosis, in which the cell engulfs large quantities of extracellular material.
What were the tumor cells after? Analysis of their metabolome indicated that procurement and breakdown of two amino acids, tyrosine and phenylalanine, dominated the metabolic changes in response to starvation. The authors noted that these two can serve as the raw material for energy production through the mitochondrial tricarboxylic acid (Krebs) cycle. When they knocked down HPDL, a central enzyme in the pathway from phenylalanine to the TCA, cell growth was significantly impaired. Blocking or reducing expression of HPDL, or the macropinocytosis promoter PAK1, reduced the ability of tumor cells to migrate and to invade surrounding tissue.
“Our results indicate that breast cancer cells take advantage of nutrients in the extracellular matrix in times of nutrient starvation, and that this process depends on both macropinocytosis and metabolic conversion of key amino acids to energy-releasing substrates,” Rainero said. “HPDL-mediated metabolism of tyrosine and phenylalanine could represent a metabolic vulnerability of cancer cells thriving in a nutrient deprived microenvironment.”
The authors add, “This study identified a novel mechanism employed by breast cancer cells to survive in the challenging environment they are in within tumours. As sources of food are scarce, cancer cells gain the ability to eat and digest components of the matrix around them. Here we have identified a key metabolic process that the cells need to be able to take advantage of the matrix, which could represent a novel therapeutic target.”
Related
The material in this press release comes from the originating research organization. Content may be edited for style and length. Want more? Sign up for our daily email.









![President Trump Gives Barron Trump A Shout Out At His Inaugural Party—Barron’s Unexpected Response is Pure Gold! [VIDEO] | The Gateway Pundit President Trump Gives Barron Trump A Shout Out At His Inaugural Party—Barron’s Unexpected Response is Pure Gold! [VIDEO] | The Gateway Pundit](https://www.thegatewaypundit.com/wp-content/uploads/2025/01/barron-trump-crowd-.jpg)