
Neuralink says it has permission to conduct its first human trials
SOPA Images Limited/Alamy
Brain-computer interface company Neuralink announced on 25 May that it has received approval from the US Food and Drug Administration (FDA) for a clinical study in humans.
Neuralink made the announcement on Twitter: “We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study.” The tweet said that the approval “represents an important first step that will one day allow our technology to help many people”.
The firm also said that the recruitment is not yet open for the trial, and it has yet to give any further details about what the trial will entail.
What is Neuralink?
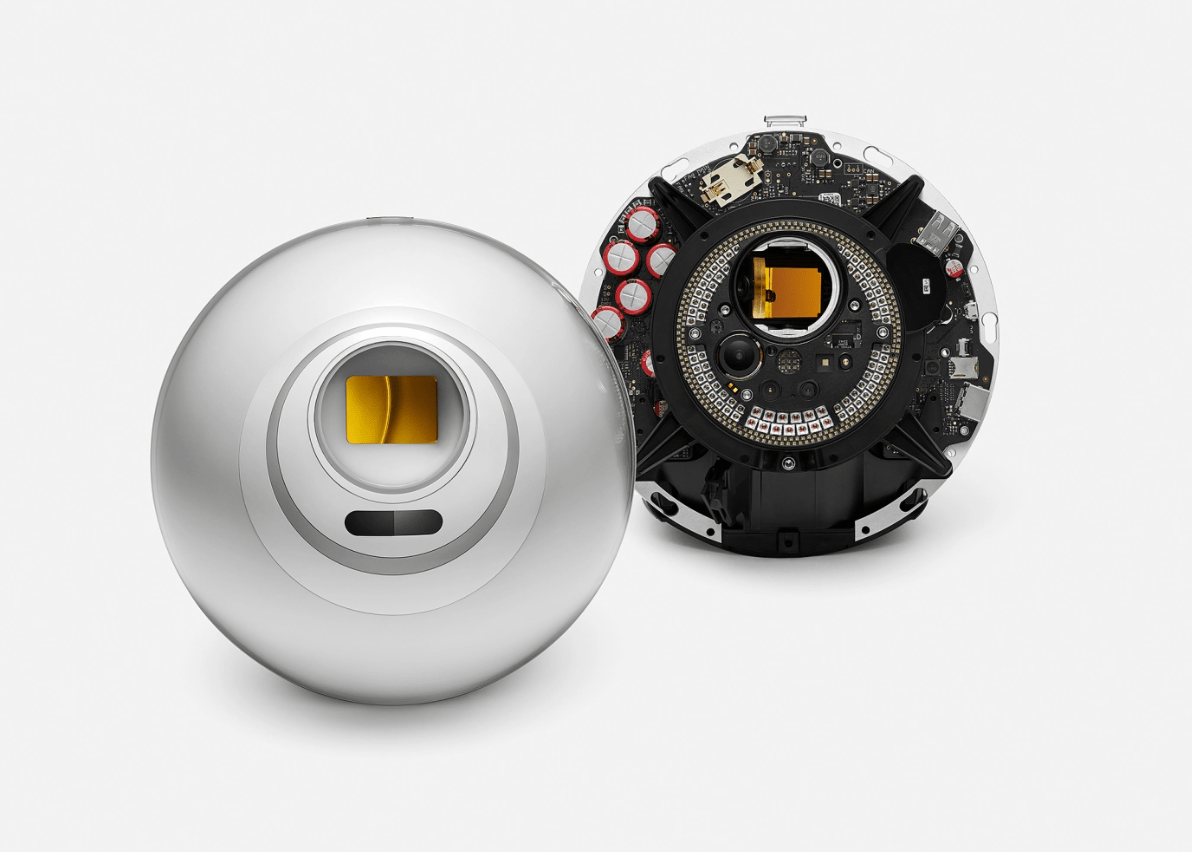
Neuralink was formed in 2016 by Elon Musk and a group of scientists and engineers with the ultimate aim of making devices that interface with the human brain – both reading information from neurons as well as feeding information directly back into the brain. So far the company has had mixed success.
In 2020, Musk showed Neuralink’s prototype brain-computer interface publicly for the first time. The prototype was shaped like a coin with wires attached to one side of it. The circular part embeds in the skull and the wires sit a few millimetres into the surface of the brain.
As part of that announcement, the Neuralink team showed a pig that had the device implanted to monitor neurons in her snout, producing signals as the animal touched her snout to food or the ground, and another that had safely had the device implanted and then removed. A year later, the company then showed how a monkey could play the classic video game Pong using its device. The company received criticism after some monkeys in the tests died and reports came out that others were treated poorly.
Similar devices for detecting and processing brain signals have been used by research labs to help people with paralysis walk again, develop a mind-controlled wheelchair and allow people with locked-in syndrome to communicate.
Neuralink did not immediately respond to New Scientist‘s request for comment.
Topics:




















































.jpg)


