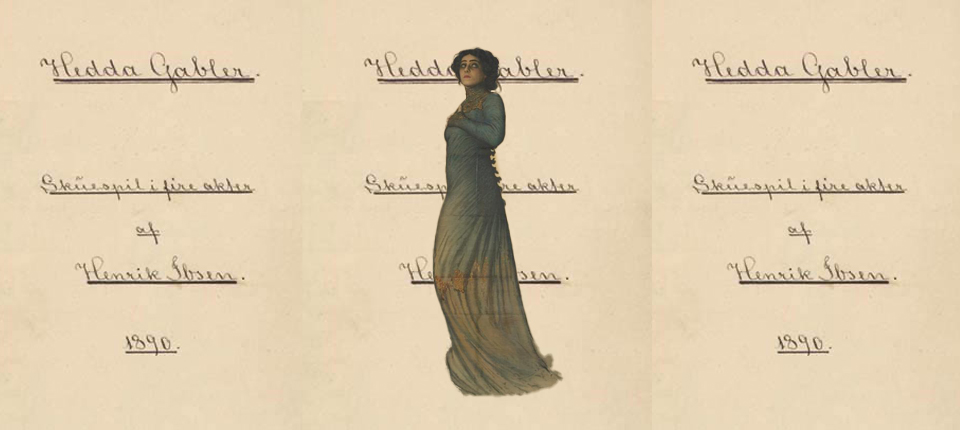
Bridge editing physically links two strands of DNA
Visual Science
A powerful form of DNA-editing machinery discovered in bacteria might allow us to make much bigger changes to genomes than is currently possible with CRISPR-based techniques. However, it isn’t yet clear whether it will work in human cells.
Patrick Hsu at the Arc Institute in California calls the new genome editor the “bridge editing” system because it physically links, or bridges, two pieces of DNA. It can be used to alter huge sections of a genome, says Hsu, whose team worked out how sequences of “parasitic” DNA in bacteria naturally use the system to replicate, and how it might be adapted for genome editing.
“We’re excited about the potential to do much broader genomic changes beyond what we can currently do with CRISPR,” he says. “We think this is an important step towards the broader vision of genome design.”
CRISPR gene editing has revolutionised biology since it was unveiled in 2012. It is being used for many different purposes, and the first CRISPR-based treatments were approved last year. However, the basic form of CRISPR, which uses the Cas9 protein, is more of a gene destroyer than a gene editor.
There are two parts to the standard CRISPR Cas9 protein. One part links up with a guide RNA molecule and seeks out any DNA that matches a certain section of the guide RNA. Because it is easy to make custom guide RNAs, this means that CRISPR Cas9 can be “programmed” to seek out any part of the genome.
The second part of CRISPR Cas9 is a cutter that severs DNA once the Cas9 has bound to its target site. The cell repairs the damage and the Cas9 cuts it again, and this keeps happening until mistakes are made during the repairs, mutating the target site in a directed way.
While being able to mutate specific sites is useful, biologists would prefer to make more precise changes, so they have been modifying CRISPR proteins to edit DNA directly instead of relying on cell repair mechanisms. Base editors, for instance, can change a single DNA letter to another without cutting the DNA. Prime editors, meanwhile, can turn an extra section of guide RNA into DNA and add it to the target site.
These modified forms of CRISPR could help treat a huge range of conditions and several human trials are already under way, but tackling some diseases requires more advanced genome alterations. Lots of teams around the world are working on ways of doing this. Some realised that the mechanism used by genetic parasites called IS110 elements to cut and paste themselves from one part of a genome to another had potential, because it is RNA-guided like CRISPR, but Hsu’s team is the first to get the complete picture of how it works.
The bridge-editing system consists of a so-called recombinase protein that hooks up with a guide RNA, like the CRISPR Cas9 protein. What makes it unique is that the guide RNA specifies two DNA sequences to seek out, not just one, Hsu’s team discovered.
One sequence specifies the target site in the genome to be altered, just as in CRISPR, while the other specifies the DNA to be altered. This system can be used to add, delete or reverse DNA sequences of virtually any length.
There are already ways of doing this, but they typically involve multiple steps and leave extra bits of DNA, called scars, behind. “Bridge editing is effectively scarless,” says Hsu. “It offers an unprecedented level of control for manipulating genomes.”
This means it could be used to do far more than simply replace faulty genes, he says. It could also help us completely reshape the genomes of plants and animals. “What we’d like to do is to move beyond inserting individual genes to do chromosome-scale genome engineering,” says Hsu.
“The discoveries reported are indeed exciting, and the underlying biology is truly remarkable,” says Stephen Tang at Columbia University in New York, but so far bridge editing has only been shown to work in bacterial cells or in test tubes. It remains to be seen whether and how well it will work in complex cells like those of humans, says Tang. But even if bridge editing fails to work in initial tests in human cells, it’s likely that in time the system can be modified so it does work
Topics: