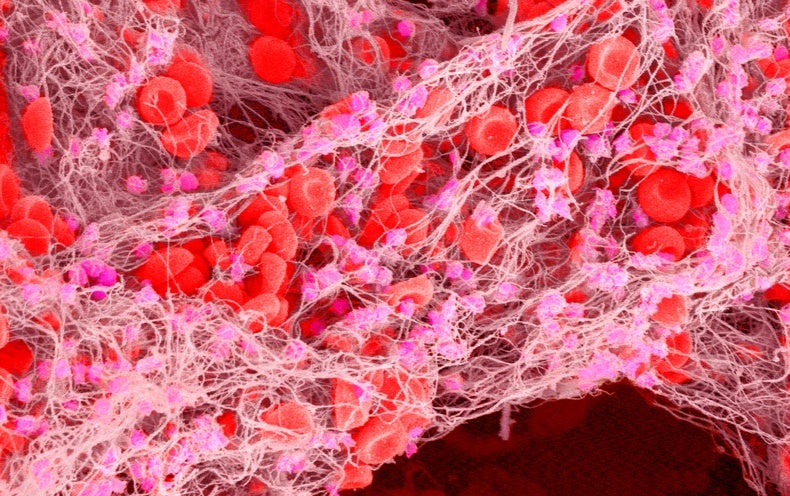
On 22 November, the US Food and Drug Administration (FDA) approved the first gene therapy for the genetic blood-clotting disorder haemophilia B—a one-time treatment that costs US$3.5 million.
Hemgenix—developed by the pharmaceutical company CSL Behring, based in King of Prussia, Pennsylvania—uses a modified virus to deliver a gene to the recipient’s liver cells. The gene codes for a protein involved in blood clotting called factor IX, which people with the disease are unable to produce.
Clinical trial data suggest that the single dose of Hemgenix will provide people with moderate to severe haemophilia with adequate protection from uncontrolled bleeding for eight years, and potentially longer.
But the treatment’s hefty price tag makes it the most expensive drug in the world. And although it seems to be effective, gene-replacement therapies for the most common form of haemophilia remain elusive.
Significant savings
CSL Behring says the cost is justified. In a statement, the company said that even at a cost of $3.5 million, Hemgenix could save the US health-care system $5 million to $5.8 million per person treated, because of its proven effectiveness at decreasing or eliminating the need for regular injections of factor IX. People with haemophilia B (who account for 15% of haemophilia cases) are currently given factor IX once or twice a week. The protein is required to form blood clots, but people with the disease lack the gene required to make it in sufficient quantities. If the condition is left untreated, people experience uncontrolled bleeding that can be life-threatening.
“Living with haemophilia is all about where one is born,” says Glenn Pierce, vice-president of the World Federation of Hemophilia in Montreal, Canada. “In the US, the treatment of an adult with haemophilia B averages $700,000–800,000 per year. The high price of Hemgenix will pay for itself in a relatively short time, and assuming it lasts.”
But scientists worry that the price would not be affordable in low- and middle-income countries, where most people with haemophilia live and where supplies of treatments and factor IX are often insufficient. “As new technologies such as gene therapy emerge on the scene, those who would benefit most can least afford to pay. We cannot leave the majority of the world behind,” says Pierce. CSL Behring declined to comment on the drug’s pricing beyond its public statement.
Promising results
The latest clinical trial of Hemgenix, which included 54 people with haemophilia B, reported a 54% reduction in the number of bleeding episodes per year, and 94% of participants discontinued any prophylactic therapy within two years of receiving the single dose. “The patients start making factor IX very quickly … in seven to eight months after the single dose, for nearly all patients, the level of factor IX had stabilized,” says Andrew Nash, CSL Behring’s chief scientific officer.
Even the lowest response in the clinical trial, a 10% increase in factor IX levels, is sufficient to prevent spontaneous bleeding, researchers say. But patients might require top-up prophylaxis treatments after injuries, or if they’re having major surgery and their factor IX levels are less than 50%.
“If you’re in the 10–40% range, you could still get a problem with major trauma or surgery. But you can pretty much forget about haemophilia,” says Edward Tuddenham, a consultant haematologist at University College London and part of the research group that designed the viral vector that CSL Behring licensed.
Tuddenham and his colleagues showed in an eight-year follow-up study of a clinical trial of a similar drug for haemophilia B that there are good reasons to consider gene therapies a stable and durable treatment.
“The approval of Hemgenix is a key milestone on the road to a cure, and it appears likely some recipients will indeed be cured for many years,” says Pierce.
Immunity issues
The FDA’s approval highlights difficulties in the quest to develop gene therapies for haemophilia more generally. Only 15% of people with haemophilia have haemophilia B. Most have haemophilia A—a genetic disorder caused by a deficiency in a different blood-clotting protein called factor VIII, which is encoded by a different gene.
Finding an effective gene therapy for haemophilia A has proved challenging, because a greater increase in factor VIII production is needed to get a good therapeutic effect, and some clinical trial participants have shown strong immune responses to the viral vector used to deliver the gene.
“In haemophilia A, there is an obvious waning off with time and [the gene expression] may only last for eight years,” says Michael Makris, who studies haemostasis and thrombosis at Sheffield University, UK. “Once you have adeno-associated viral gene therapy, you make antibodies to the AAV vector, so you cannot have it again.”
On 24 August, the European Medicine Agency approved a gene therapy for haemophilia A by BioMarin Pharmaceutical, based in San Rafael, California. After rejecting their first application, the FDA is now considering BioMarin’s resubmission.
“Gene therapy—while exciting and promising—should not be considered lightly,” says Leonard Valentino, a former haemotologist who is chief executive of the US National Hemophilia Foundation in New York City. “It is a potentially life-changing decision, and with any life-altering decision, there can be positive and negative effects”.
This article is reproduced with permission and was first published on December 6 2022.