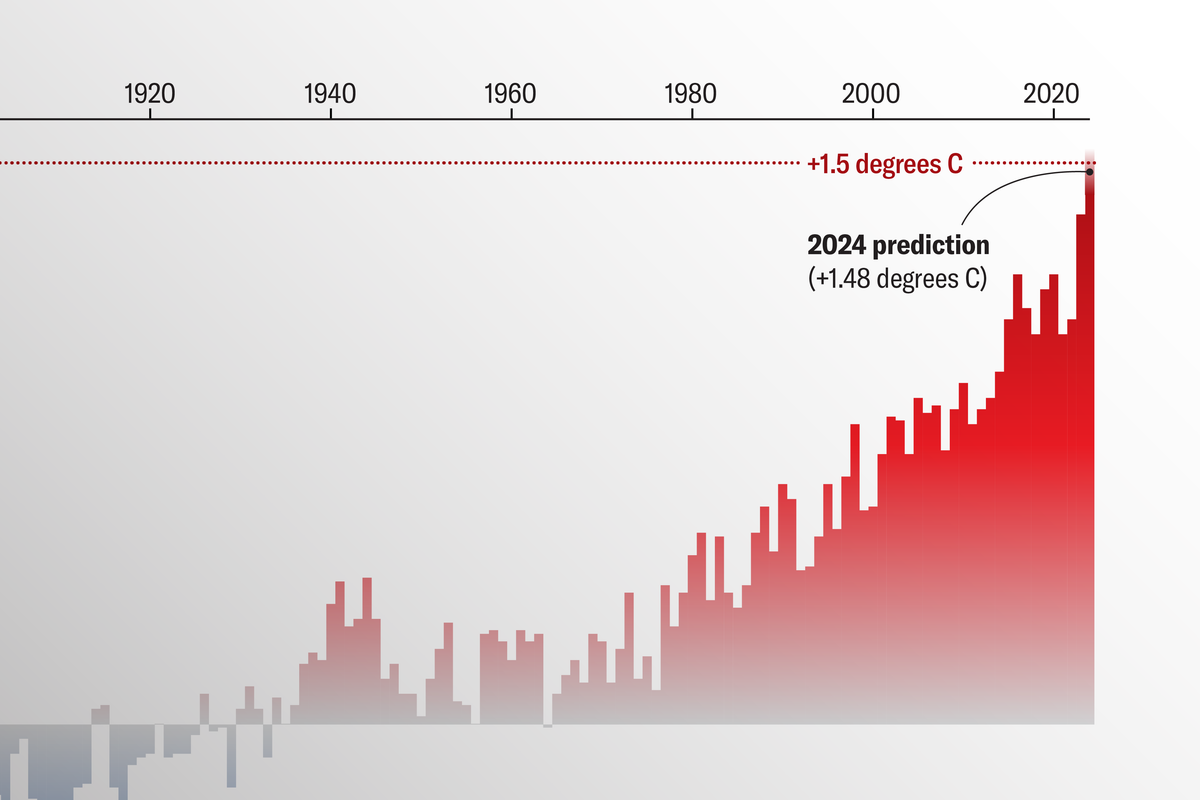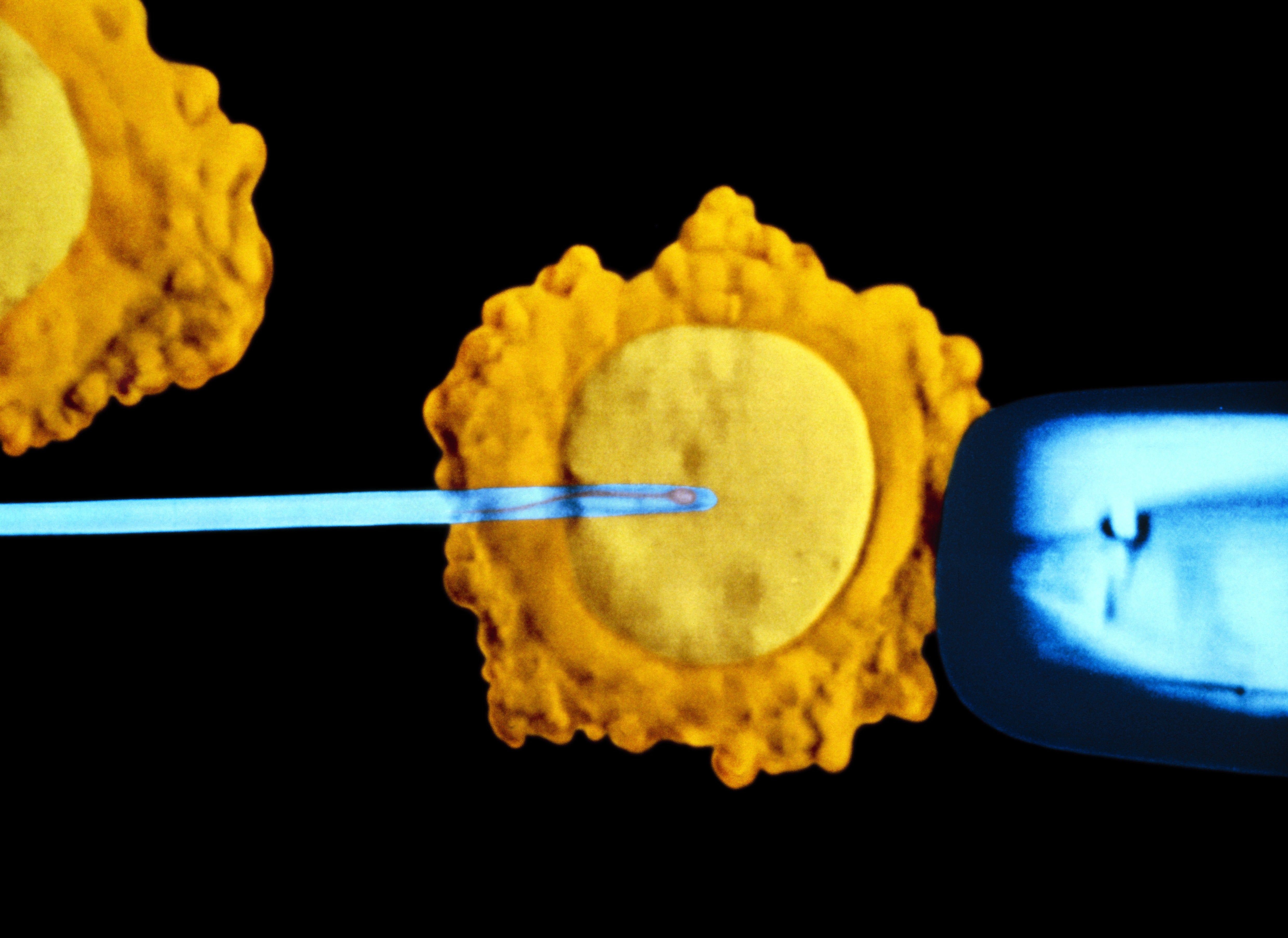
In clinics today, fertility patients using in vitro fertilization (IVF) are routinely advised to pay for an expensive supplemental test called preimplantation genetic testing for aneuploidy (PGT-A), in which a handful of cells are removed from the embryo to examine their DNA. For those who can afford it, PGT-A is popular because it can flag genetic abnormalities that increase the odds that a pregnancy, should it occur, will end in miscarriage.
Pervasive use of the test has also generated controversy. The authors of an April 2022 study in Human Reproduction have sparked debate and alarmed prospective parents by suggesting that many clinics are too quick to discard embryos based on PGT-A and are ignoring a growing body of evidence that some of these embryos are capable of producing a viable pregnancy.
If all of a patient’s embryos are rejected based on PGT-A results, they may lose their only chance at taking home a baby or be directed prematurely toward expensive alternative technologies such as donor eggs that would deprive them of a child genetically related to both parents. In a quote in a 2017 article in New York Magazine’s the Cut, study co-author Norbert Gleicher of the Center for Human Reproduction in New York City called this waste of potentially good embryos “an unprecedented scandal.”
Proponents of the test have pushed back strongly on this criticism. They say that PGT-A benefits decision-making for anyone who can afford it and provides patients with realistic information on each embryo’s odds of viability. PGT-A is also credited with reducing the risks and costs associated with earlier iterations of IVF.
The process of halving and then combining maternal and paternal chromosomes is a delicate operation that often goes awry, and as a result, embryos frequently have added or missing DNA—a condition known as aneuploidy, which can prevent or derail a pregnancy. Aneuploidy is well established as the most common cause of miscarriage in the first trimester and helps explain why many couples who place all their hopes on implanting a single egg each cycle are unable to have a baby. IVF uses artificial hormonal stimulation to coax the ovaries into ripening multiple eggs that are then removed and fertilized in the lab to produce as many embryos as possible. PGT-A was developed to give doctors a better basis for embryo selection than eyeballing them under a microscope, which is a hit-or-miss form of assessment that leans heavily on gut instinct.
For those patients with plenty of embryos, the advantages of PGT-A are clear. Ruling out the embryos most likely to result in miscarriage helps reduce the number of IVF cycles required to achieve a successful pregnancy. Every IVF cycle is expensive and, like miscarriage, physically and emotionally taxing. What’s more, according to Teresa Cacchione, a genetic counselor at Reproductive Medicine Associates of New York, it is the use of PGT-A that has enabled a recent shift in practice in favor of transferring only a single embryo per cycle into the uterus rather than two or more. This change has radically reduced the high number of twins, triplets and higher-order multiples that for decades represented the primary source of increased medical risk for babies conceived through IVF.
But while the rationale for use of PGT-A appears sound, research has exposed the limitations of embryo biopsy. As lead author David Barad of the Center for Human Reproduction points out, the cells biopsied are a small sample of the whole. They are pulled from the tissue that will eventually form the placenta and not the fetus itself. “If you reach down in a field full of wildflowers and close your eyes and pull up three flowers, and they’re all blue, that doesn’t mean the field is all blue,” he says. Yet other studies have shown that PGT-A does a good job at representing the mix of cells in the embryo.
What testing has shown is that many embryos—perhaps even all—are in fact a mix of different cell lines with variations in their DNA complement. Mistakes occur as cells divide and multiply. Most of these are lethal, but surviving cells will pass down whatever changes occurred to all their daughter cells, creating a sort of alternative genetic lineage. This mix of cell lines is called mosaicism. A 2020 American Society for Reproductive Medicine (ASRM) paper estimated that the odds of reproductive success are inversely related to the level of mosaicism identified in an embryo.
In practice, embryos are classified as fully aneuploid rather than mosaic when more than 80 percent of the cells biopsied show one or more genetic abnormalities. Cacchione says that her facility will not transfer fully abnormal embryos at this point “because of the incredibly low likelihood of an ongoing pregnancy and the very high chance of loss.” Reproductive Medicine Associates of New York will offer prospective parents the option of transferring embryos identified as mosaic, but some clinics will not, despite guidance from organizations such as ASRM that this can be done with appropriate counseling.
In their study, Barad and his co-authors transferred both mosaic and fully aneuploid embryos after patients were denied the opportunity to use them at other clinics. Their findings, in line with previous work, demonstrate that mosaic embryos are frequently capable of generating a successful pregnancy. Interestingly, in follow-up testing of a fetus or subsequent child, the rogue cell lines with added or missing DNA have often disappeared altogether.
This resilience in the embryo has taken some by surprise. Experts hypothesize that embryos can self-correct when healthy cell lines outperform the competition, pushing the aneuploid cell lines into obsolescence. “It didn’t surprise me at all,” says Jamie Grifo, director of the NYU Langone Fertility Center, “because we knew from earlier experience that any embryo has a shot.” But long shots have high costs. Grifo posits that it might take more than 125 transfers of fully aneuploid embryos to get a single pregnancy; all of those other transfers represent failed cycles of IVF, including an estimated 35 to 40 miscarriages. Though Barad champions the use of both mosaic and fully aneuploid embryos, the differences between the two in his own data were stark: 23 mosaic embryos transferred produced six live births, while 79 fully aneuploid embryos produced only two.
Lurking in the background is the fear that using aneuploid or mosaic embryos could produce children with serious medical issues. Cacchione says she acknowledges the issue with patients. “We don’t have long-term data,” she says. “Most of the babies born from known mosaic transfers are under the age of four.” But she points out that doctors were undoubtedly transferring mosaic embryos unwittingly for decades before the routine use of PGT-A, with no evidence of increased birth defects. “That’s all very reassuring,” Cacchione says. Still, Barad suggests, concerns about malpractice may limit clinics’ willingness to allow patients to try and use any embryos deemed “abnormal.” “Some institutions are being guided by their lawyers,” he explains.
Barad claims that aggressive marketing of PGT-A, which typically adds $4,000 to $5,000 to the cost of IVF, may be resulting in overuse. But Cacchione contends the test is valuable for any patient who can afford it, provided it is combined with good counseling and patient education. She argues that PGT-A allows patients to weigh a realistic understanding of the chance of a successful pregnancy against the cost of repeated rounds of IVF and the physical and emotional toll associated with miscarriage. In the end, Cacchione says, “it’s a very personal decision.”